金桔
金币
威望
贡献
回帖0
精华
在线时间 小时
|
登陆有奖并可浏览互动!
您需要 登录 才可以下载或查看,没有账号?立即注册


×
泛说前沿系列学术栏目,致力关注肿瘤精准医疗及其转化医学研究,剖析领域发展,解读前沿文献,深挖研究思路,磨砺分析工具,与您一起追踪前沿。
成像技术和组织活检是头颈肿瘤诊断中常用的检测手段,可通过磁共振成像、电子计算机断层扫描以及正电子发射型计算机断层显像等方式进行病灶扫描诊断。对于无法成像的部位,一般使用超声引导的细针抽吸器对病灶进行取样。但是组织取样存在异质性高、面容损伤等缺陷[1]。此外,大部分头颈肿瘤患者被发现时病程往往已经发展到中晚期,只有不到三分之一的患者可以在早期被发现并进行治疗[2]。而早期的图像技术也不能满足早期头颈肿瘤的转移和预后监测的需求。因此,寻找一种入侵性较小的检测方式来监测头颈肿瘤的发生和转移从而提高患者的生存率迫在眉睫。液态活检能够通过检测唾液、血液中的肿瘤循环细胞(CTCs)、肿瘤循环DNA(ctDNA)以及外泌体,实现实时监测肿瘤发生发展的目的[3]。与常规的成像技术和组织活检相比,液态活检在实时追踪肿瘤发展进程以及转移情况等方面存在巨大优势,且侵入小不造成二次创伤,有望成为头颈肿瘤日常监测的工具之一。本文对液态活检在头颈肿瘤中的研究进行了概述。
1. 头颈肿瘤液态活检与组织活检的一致性
Applicability of liquid biopsies to represent the mutational profile of tumor tissue from different cancer entities
Journal: Oncogene
IF: 9.867
DOI: 10.1038/s41388-021-01928-w 该研究共招募了18名转移性头颈鳞癌(HNSCC)、结直肠癌(CRC)以及黑色素瘤(MEL)患者(每个癌种各6人)。研究人员对HNSCC患者的血液样本和组织样本采集之后,使用RosetteSep™ Human CD45 Depletion kit进行CTCs分离,并通过EpCAM和EGFR标签对HNSCC来源的CTCs进行鉴定。基于已知的HNSCC突变基因panel[4],进行外显子组测序。Germline、FFPE、cfDNA和CTCs的RAW data测序深度分别是158倍、83倍、74倍和47倍。其中HNSCC01.1和HNSCC02.1样本属于high-input样本(cfDNA>30ng),而HNSCC03.1、HNSCC04.1和HNSCC05.1为low-input样本(cfDNA<30ng)(HNSCC06.1组织样本并没有检测到相关突变,因此没有进行组织突变与血液突变一致性的分析)。结果显示当cfDNA的总量大于30ng,cfDNA突变与组织突变检测结果的一致性更高,约为50%(图1-2)。当cfDNA总量<30ng,cfDNA中突变检出个数以及与组织突变的一致率都会降低(图3-4)。
在high-input cfDNA的HNSCC01.1和HNSCC02.1组织样本与cfDNA同时检测到的突变有ACACA、ATR、LAMA2、PIK3CA、SMARCA4;ARID1A、EYA1、INPP5D、KCNT2、NEUROD6是组织中特异性突变(图1)。研究人员认为部分突变基因从组织进入循环系统后到进行液态活检的这段时间以几何倍数扩增,从而在血液中被检测到,而在组织中无法检测。通过组织样本反向验证血液中的基因突变,结果显示组织中也能检测到的cfDNA突变有EZH2、LAMA2、PIK3CA、SMARCA4;EGFR、HFM1、KDM5B、LTBP1、MYH10是cfDNA特异性突变(图2)。这些结果表明液体活检能够用来辅助和参与检测肿瘤的相关突变。
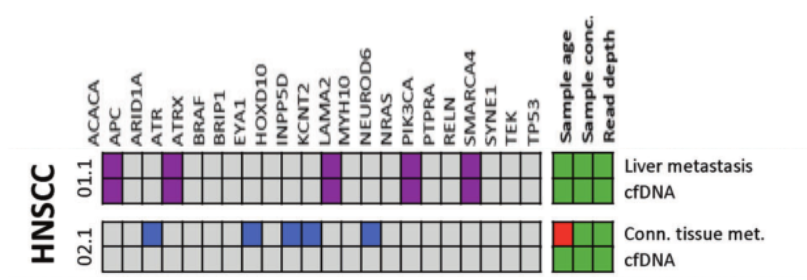
图1 组织突变在high-input cfDNA的分析。紫色方块:HNSCC患者组织和血液中共有的突变;蓝色方块:HNSCC患者组织中特有的突变
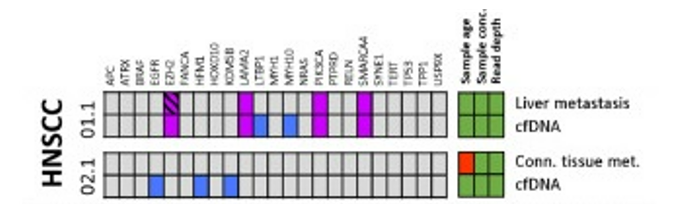
图2 high-input cfDNA突变及其在组织样本中的分析。紫色方块:HNSCC患者血液和组织中共有的突变;蓝色方块:HNSCC患者cfDNA中特有的突变。
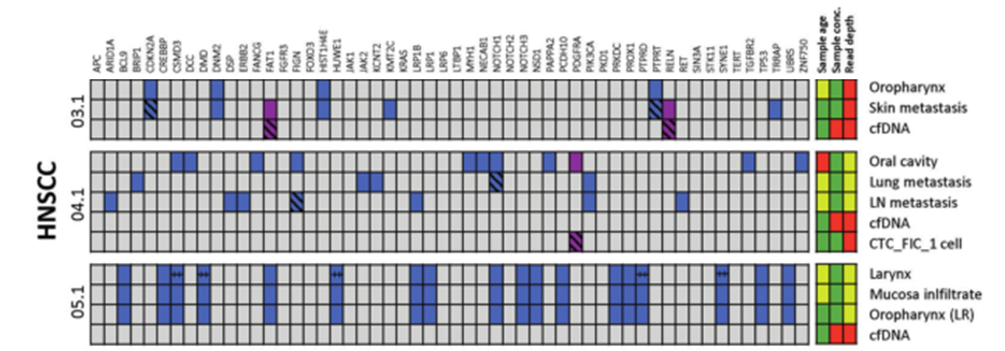
图3 组织突变在low-input cfDNA的分析。紫色方块:HNSCC患者组织和血液中共有的突变;蓝色方块:HNSCC患者组织中特有的突变。
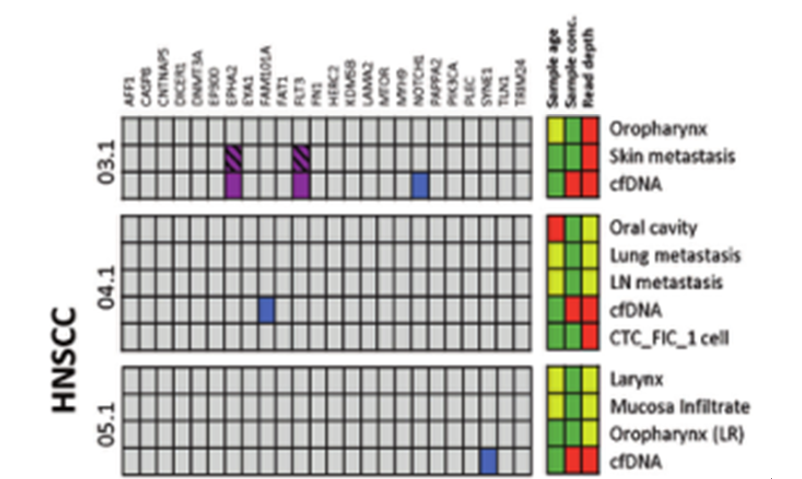
图4 low-input cfDNA突变及其在组织样本中的分析。紫色方块:HNSCC患者血液和组织中共有的突变;蓝色方块:HNSCC患者cfDNA中特有的突变。
2. CTCs对化疗和西妥昔单抗治疗后的HNSCC患者预后的预测作用
Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: The CIRCUTEC prospective study
Journal: Clinical Chemistry
IF: 8.636
DOI: 10.1373/clinchem.2019.305904 这项前瞻性多中心研究评估了CTCs对化疗和西妥昔单抗治疗后复发不可手术或转移性头颈部鳞状细胞癌(rHNSCC)患者预后的预测作用。该研究采集了65例HNSCC患者治疗的第0天(D0)、第7天(D7)和第21天(D21)的外周血样本,用CellSearch®、EPISPOT和流式细胞术(FCM)分别检测患者血液中的CTCs。研究人员发现在D0期,EPISPOT、CellSearch和FCM分别在69%(45/65)、21%(12/58)和11%(7/61)的患者中检测到CTCs。其中,EPISPOT分别在D0、D7和D21检测出92%(36/39)、92%(35/38)和90%(25/28)的阳性样本。通过分析D0到D7 EPISPOTEGFR阳性患者的预后情况,发现与阴性患者相比,CTCs阳性患者PFS显著降低(3.9个月vs 6.2个月,P=0.0103)(图1)。此外,将三种检测手段两两组合检测D7期的CTCs并对患者的预后进行评估。3组结果都显示CTCs检测阳性的患者与阴性患者相比,PFS显著降低(EPISPOT/Cell Search:37/51,P=0.0311;EPISPOT/FCM:38/54,P=0.0480;Cell Search/FCM:11/51,P<0.0005)(图2)。该研究证实液态活检实时检测CTCs可用于监测rHNSCC化疗的早期反应。
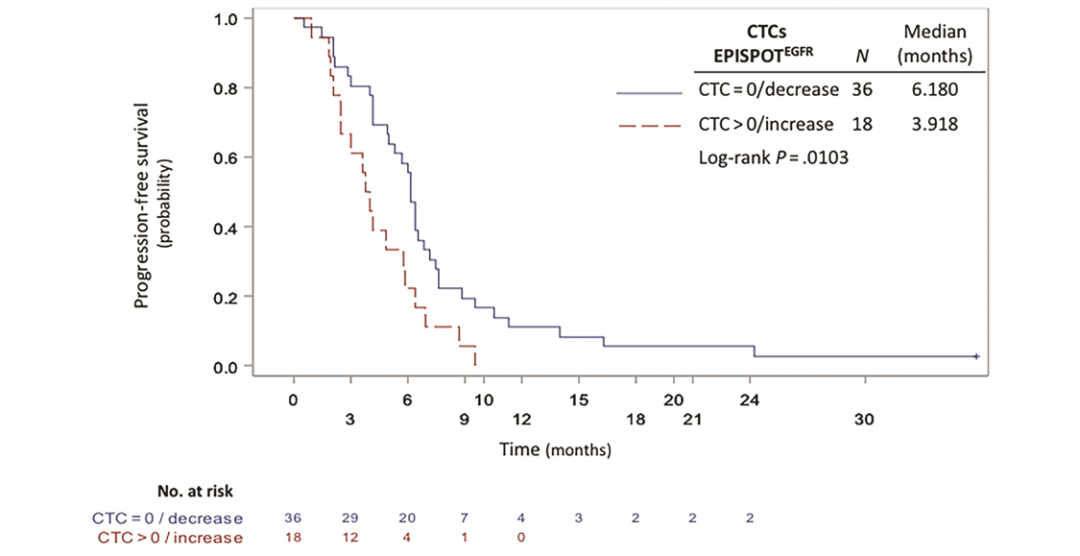
图1 复发/转移性HNSCC患者的PFS与CTCs(D0-D7)的相关性。采用EPISPOTEGFR法根据D0与D7间CTCs计数差异将患者分为2组:组1(D0与D7期间无CTCs或CTCs数量减少,蓝色实线)和组2(D0与D7间CTCs数量增加/稳定,红色虚线)
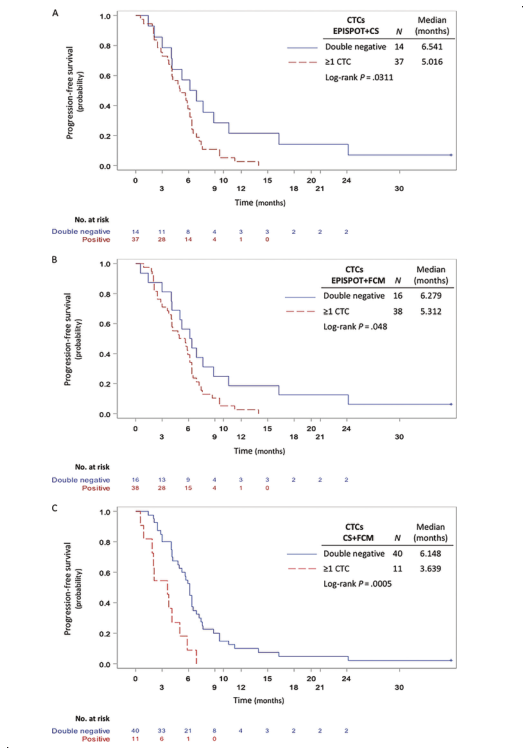
图2 复发或转移的HNSCC患者的PFS与D7的相关性。A)第一个化疗周期(D7)后EPISPOT结合CellSearch检测(n=51);B)EPISPOT结合FCM检测(n=54);C)CellSearch结合FCM检测(n=51)。双阴性:两种方法均未检出CTCs;阳性:1种方法或两种方法CTCs≥1。
3. ctDNA在预测晚期头颈肿瘤复发中的作用
Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)—a personalized circulating tumour DNA analysis in head and neck squamous cell carcinoma
Journal: British journal of cancer
IF: 7.64
DOI: 10.1038/s41416-022-01716-7 该研究纳入17例年龄在48-78岁的III-IVb期的HNSCC患者,患者P16阴性并计划手术切除瘤体。在术中采集组织样本,术前以及术后采集血液样本,并建库测序分析患者ctDNA的水平(图1)。首先基于患者特异性的组织突变结合常见突变设计患者个性化的检测模型[5],并基于此模型对患者进行ctDNA检测并判断患者是否为ctDNA阳性。通过对患者基线期和术后的CTCs分析,术后ctDNA阳性的患者相较于阴性患者更易复发(图2)。
在整个队列中,患者随访的中位时间为371天(292-532天,病例12失访不计入统计)。通过实时监测患者的ctDNA,发现ctDNA阳性患者出现肿瘤复发(图3)。Patient14的任何检测时间点都未发现pT1口腔癌相关的ctDNA,但在pT4a喉癌相关的ctDNA在术后的所有时间点都能检测到(图4a),从ctDNA检测阳性到出现临床进展的时间间隔为108天。Patient13从检测到ctDNA到临床复发为110天(图4b)。Patient9术前检测到ctDNA,术后3天以及13天检测ctDNA阴性。但在术后21天ctDNA复阳,在完成辅助治疗后ctDNA转阴。在第一次手术收的128天ctDNA含量再次上升。第二次手术后ctDNA水平转阴(图4c)。Patient2术前检测到ctDNA,术后7天ctDNA检测为阴。ctDNA水平在术后第17天重新转阳。中间患者进行辅助治疗,但是到第一次术后的253天ctDNA仍为阳性,最终在270天出现临床复发(图4d)。Patient6术前检测到ctDNA,但在术后6、21以及辅助治疗后90天内均未检测到ctDNA。在第一次术后132天,即临床进展前160天检测到ctDNA水平升高,在术后244天检测ctDNA仍为阳性以及术后292出现临床复发(图4e)。这些结果表明ctDNA能够作为监测HNSCC患者早期复发的生物标志物。
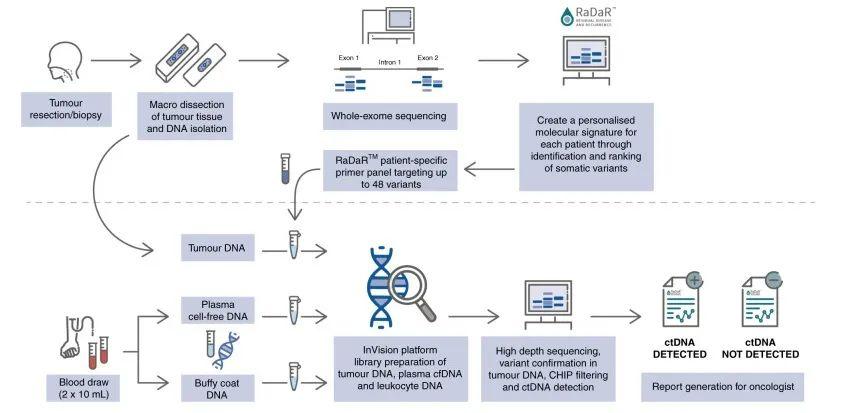
图1 RaDaRTM工作流程图。手术切除的肿瘤组织通过全外显子组测序以识别体细胞突变。为每个患者开发了个性化的ctDNA检测panel。
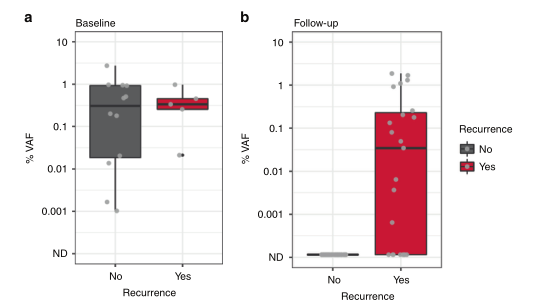
图2 估计变异等位基因频率中位百分比(%VAF)。a)术前基线样本的ctDNA水平在0.001%到2.737%之间,估计变异等位基因频率(%VAF)。b)在术后样本中,ctDNA检测水平低至0.0006%VAF,20%的ctDNA阳性样本检测水平低于0.01%VAF。
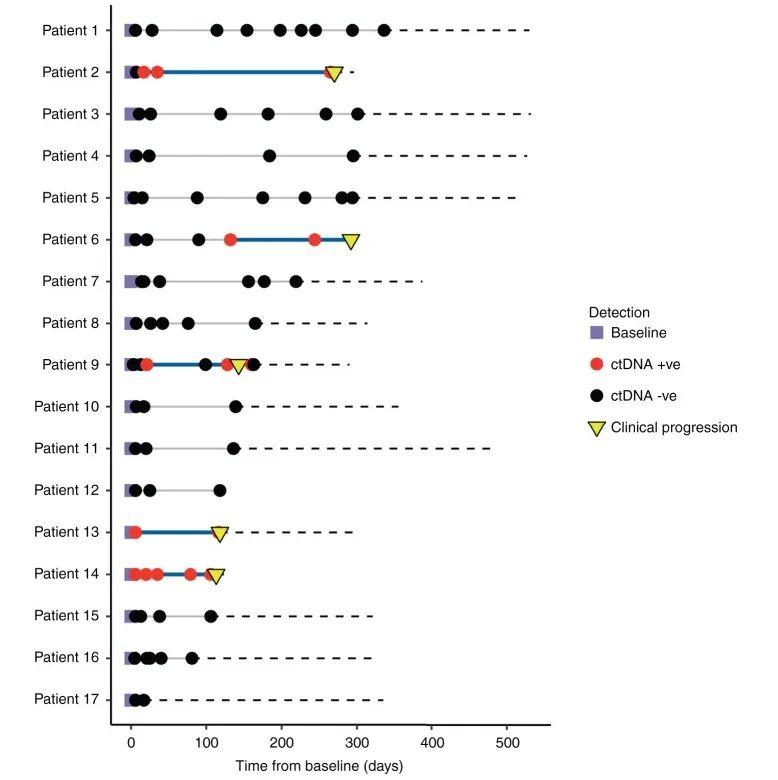
图3 纵向监测17例患者的连续血浆样本。红圈:ctDNA阳性;黑圈:ctDNA阴性;黄色倒三角:复发时间点;蓝色实线:领先时间,即从第一次检测ctDNA阳性的术后样本到临床确认疾病复发的时间间隔;黑色虚线:患者的随访总时间。
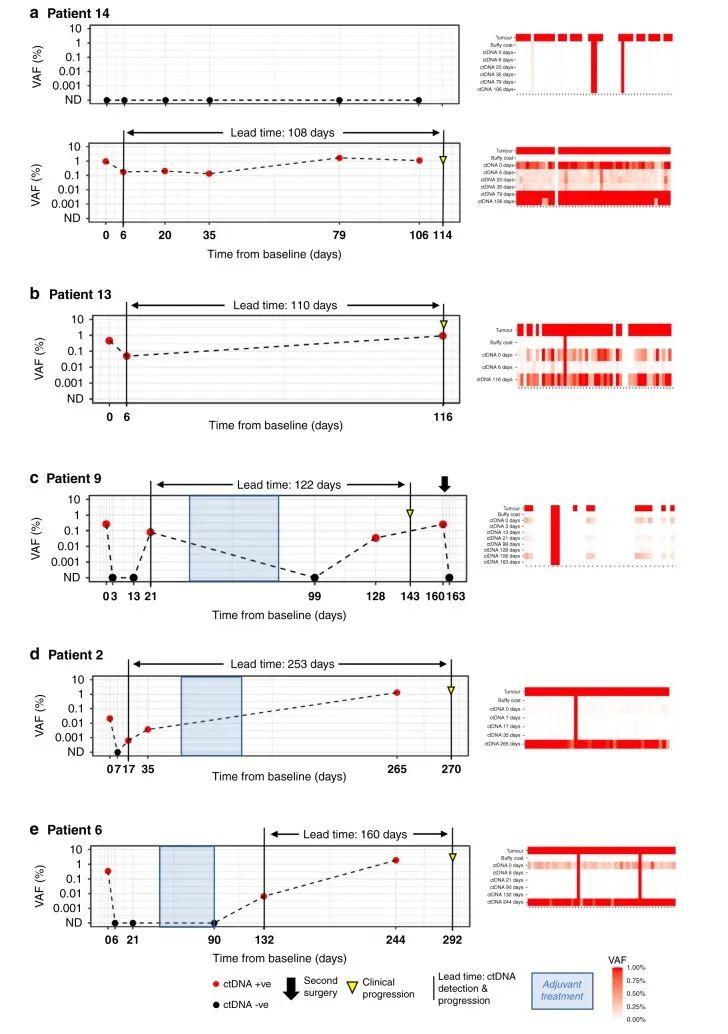
图4 5例复发患者的ctDNA纵向监测。图右侧的热图表明不同的突变。每一列表示不同的变体,每一行表示不同的样本类型。红圈:ctDNA阳性;黑圈:ctDNA阴性;黄色倒三角:临床进展;蓝色阴影部分:辅助治疗(放疗)的周期;基线(x轴)上时间点0为术前血浆采集时间点。
4. CTCs中的PD-L1水平在预测头颈肿瘤患者预后中的作用
Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma
Journal: Annals of oncology
IF: 18.274
DOI: 10.1093/annonc/mdx206 CTCs的分子特征在分子靶向治疗研究中至关重要,而CTCs中的PD-L1与患者疗效目前还鲜有报道。在这项头颈鳞状细胞癌(HNSCC)的前瞻性队列研究中,研究人员评估是否可以在HNSCC病人的基线期和治疗结束期两个不同时间点检测到CTCs中PD-L1表达,并用于预测治疗后的临床效益。该研究开发了一种针对EpCAM+ CTCs中PD-L1 mRNA表达高度敏感、特异性和匹配度较高的RT-qPCR方法。
在113例局部晚期HNSCC患者的前瞻性队列中,有94例患者的血液样本在基线期可检测到CTCs,随后经过2个周期的诱导化疗(第6周)和同步放化疗结束(第15周),有54名患者的CTCs能够被检测。结果显示24/94名基线期患者(25.5%)、8/34名诱导化疗后的患者(23.5%)以及12/54名治疗结束期患者(22.2%)的PD-L1 表达阳性。结果显示基线期CTCs PD-L1阴性的患者和阳性的患者的无进展生存期(P=0.761,图1A)和总生存期没有显著差异(P=0.235,图1B)。而治疗结束期CTCs PD-L1仍为阳性的患者无进展生存期(P=0.001,图1C)和总生存期(P<0.001,图1D)相较于CTCs PD-L1阴性的患者显著减少。这些结果表明PD-L1与HNSCC患者放化疗治疗后的生存预后有显著相关性。多因素Cox回归分析HNSCC患者CTCs PD-L1显著影响患者OS和PFS(HROS=4.07,HRPFS=7.96),并且独立于患者的年龄和性别、吸烟行为、饮酒、肿瘤TNM分期、淋巴结状态、原发部位和组织学分级(图2A,B)。
此外,该研究对18名基线检查CTC PD-L1阳性的患者的完全缓解率(CR)统计发现,7例(38.9%)在治疗后获得CR,而在41例PD-L1阴性的患者中,27例(65.9%)在治疗后获得CR(Fisher精确检验,P=0.085)。随后对治疗结束期PD-L1阳性的10名患者中分析发现只有2名患者(20%)获得CR,而PD-L1阴性的35名患者中,有28名(80%)获得CR(Fisher精确检验,P=0.001),表明治疗结束期的PD-L1阳性与患者完全缓解密切相关(OR=16,95%CI¼2.76-92.72,P=0.002)。这些结果证明检测CTCs中的PD-L1水平可能能够为HNSCC患者提供重要的预后信息,并且治疗结束后仍检测到CTCs PD-L1的HNSCC患者可以进一步使用PD-L1抑制剂进行治疗。
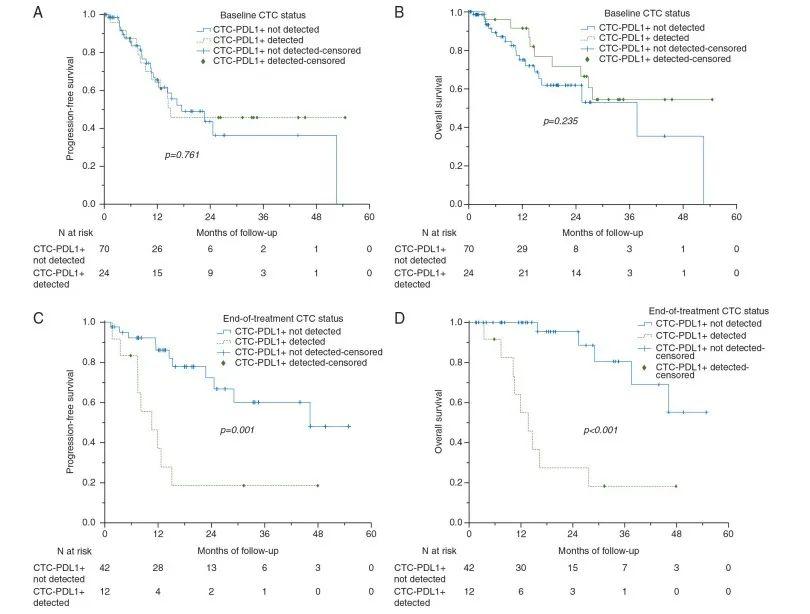
图1 A)基线期PD-L1阴性和阳性患者的OS没有显著差异(P=0.761);B)基线期PD-L1阴性和阳性患者的PFS没有显著差异(P=0.235);C)治疗结束期PD-L1阴性和阳性患者的OS具有显著差异(P=0.011);D)治疗结束期PD-L1阴性和阳性患者的PFS具有显著差异(P=0.004)。
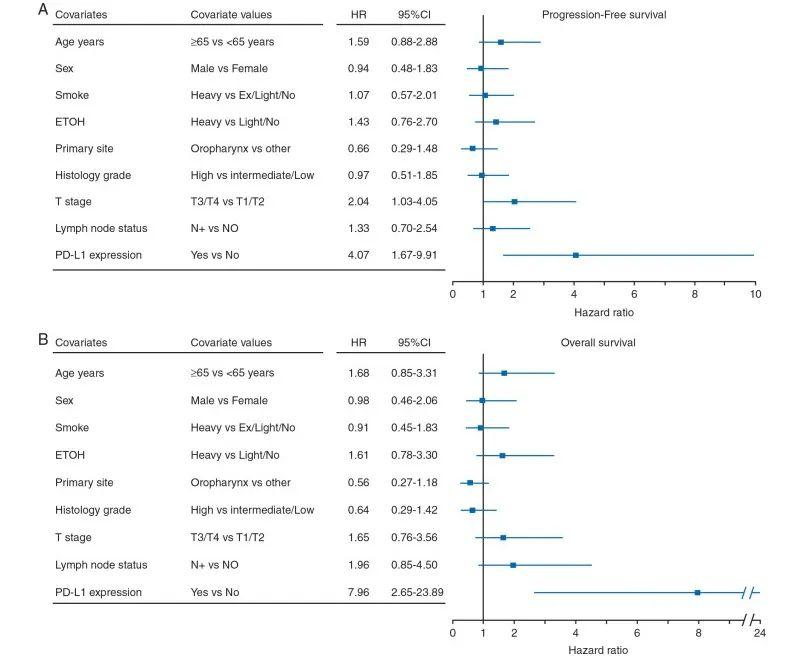
图2 治疗结束期HNSCC患者OS(A)和PFS(B)的多因素Cox模型森林图。
5. ctHPV水平在预测头颈肿瘤患者预后中的作用
Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer
Journal: Journal of clinical oncology
IF: 44.544
DOI: 10.1200/JCO.19.02444 血液中游离肿瘤人乳头瘤病毒DNA(human papillomavirus DNA,ctHPV DNA)是人乳头瘤病毒相关口咽鳞状细胞癌(OPSCC)敏感且特异的生物标志物[6]。因此ctHPV在预测口腔癌患者复发方面可能有着重要作用。该研究对115例非转移性的HPV+(P16阳性)口腔癌患者(75%的患者为早期患者)治疗后的ctHPV进行纵向监测。所有患者在接受治疗性放化疗(CRT)后3个月接受正电子发射断层扫描/计算机断层扫描,此后每2-4个月(1-2年)进行临床评估,然后每6个月(3-5年)进行临床评估,每6个月做一次胸部影像学检查,每6-9个月采集一次血样,使用多元数字聚合酶链反应(ddPCR)分析血浆ctHPV DNA水平。主要观察终点是ctHPV的阴性预测值(NPV)和阳性预测值(PPV)。中位随访时间为23个月(范围6.1-54.7个月)。
87例患者在所有治疗后检测不到ctHPV(图1A),且无临床复发(NPV=100%;95% CI=96%至100%)。28例患者在治疗后监测中出现ctHPV阳性(图1B),其中16例患者连续2次ctHPV DNA血液检测呈阳性,经组织活检证实有15名患者复发(图2A)。两个人群连续两次ctHPV血液检测阳性的PPV为94%(95% CI,70%-99%)。ctHPV阳性和活检证实复发之间的中位提前时间为3.9个月(范围为0.37-12.9个月)。有8名患者在初始ctHPV检测阳性后,进一步追踪实时监测发现ctHPV降低,且无临床复发(图2B)。此外,ctHPV阳性的患者的无复发生存期、局部无进展生存期以及无远端转移相较于ctHPV阴性患者都显著降低(P<0.001,图C-E)。基于HPV阳性在提示口腔癌患者的高复发以及远端转移中的作用,对ctHPV阳性患者进行监测,能够及早发现这类病人的病情进展,提高这类患者的预后生存期。通过合适的治疗手段,有助于尽早对患者展开挽救性治疗。
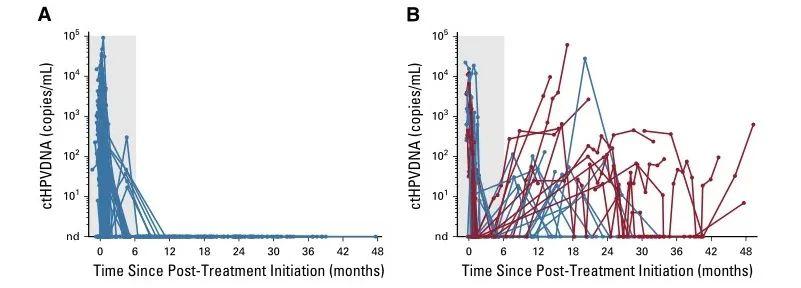
图1 ctHPV纵向监测明确高复发风险人群。A)87名患者在CRT治疗后ctHPV检测呈现阴性;B)28名患者在治疗后ctHPV检测呈阳性。
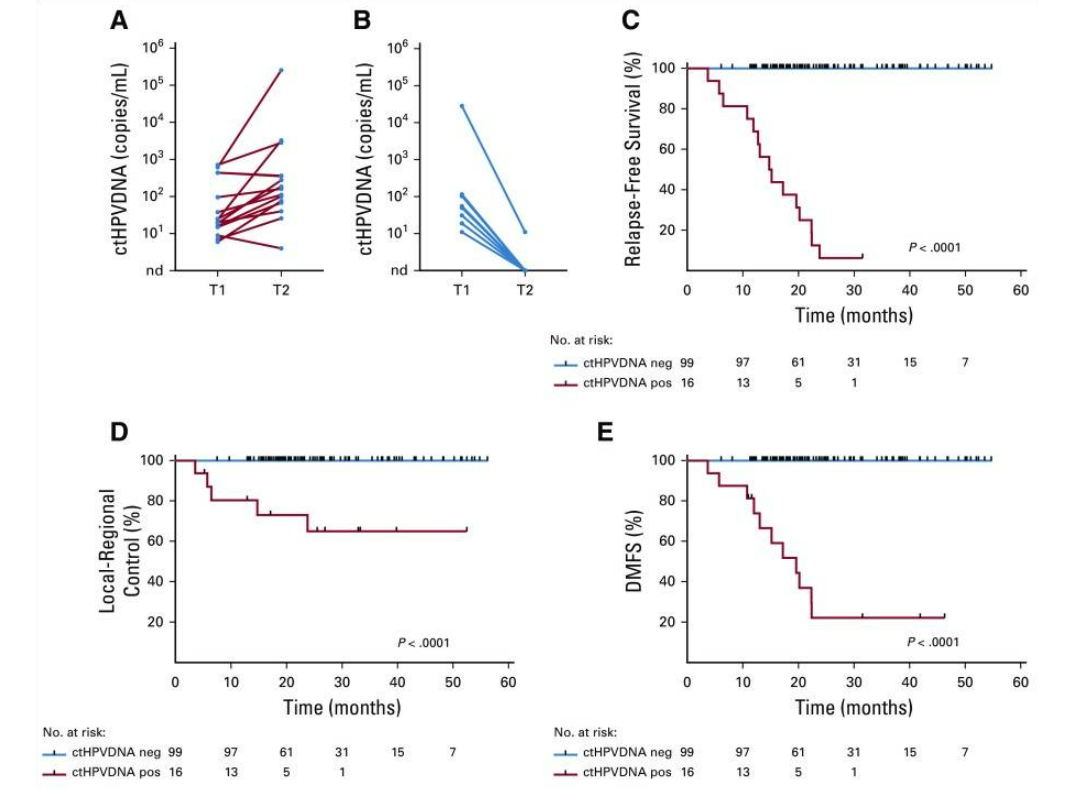
图2 ctHPV水平与患者的复发和预后的相关性。A)表示复发的15个病人连续两次检测血清中的ctHPV含量是呈现升高的趋势;B)表示未复发的8个病人,连续两次检测血清中ctHPV含量是呈现减少的趋势;C-E)分别为ctHPV阳性和阴性的患者的无复发生存期、局部无进展生存期以及无远端转移生存期。
6. cfEBV在预测或诊断早期鼻咽癌患者中的作用
Analysis of plasma epstein-barr virus DNA to screen for nasopharyngeal cancer
Journal: The New England journal of Medicine
IF: 70.670
DOI: 10.1056/NEJMx180004 鼻咽癌的发病机制与Epstein-Barr病毒(EBV)密切相关,而血液中的cfEBV DNA已被证实为鼻咽癌的生物标志物[7]。该研究通过一项前瞻性研究来调查血液中的cfEBV DNA是否有助于筛查无症状的早期鼻咽癌患者。在20174名参与者中一共有1112名参与者的血液样本中可检测到EBV DNA,其中有309/1112名患者的样本cfEBV DNA检测持续阳性。通过进一步的内窥镜和MRI检查,发现有34人患有鼻咽癌。该筛查与2013年香港鼻咽癌筛查相比,I期或II期鼻咽癌患者的比例明显提高(71%对20%,卡方检验P<0.001,图1)。CfEBV筛查出的鼻咽癌患者与香港常规筛查出的鼻咽癌患者相比,其3年无进展生存率显著提高(97%对70%;风险比=0.10;P<0.001,图2)。
在检测后1年内,19865名血浆EBV DNA呈阴性的参与者中仅有1名被检出患有鼻咽癌。血液EBV-DNA筛查鼻咽癌的敏感性和特异性分别为97.1%和98.6%(表1)。结果提示检测血液中的EBV水平相较于常规筛查能够检测出更多的早期鼻咽癌患者,帮助鼻咽癌患者的早期预防和诊断,从而大大改善鼻咽癌患者的生存质量。
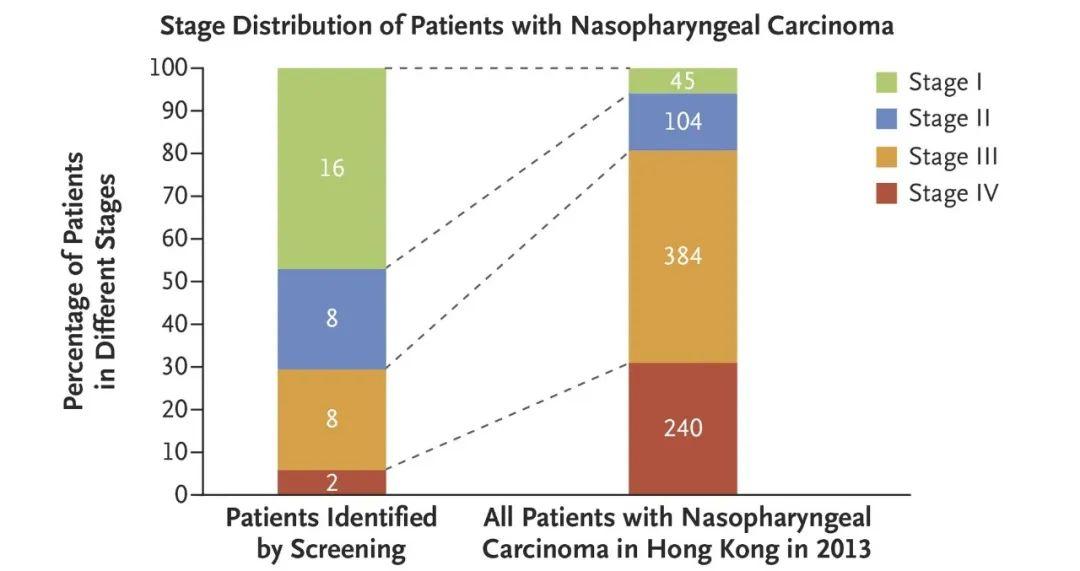
图1 34例鼻咽癌患者的肿瘤分期分布和2013年香港鼻咽癌患者的分期分布。
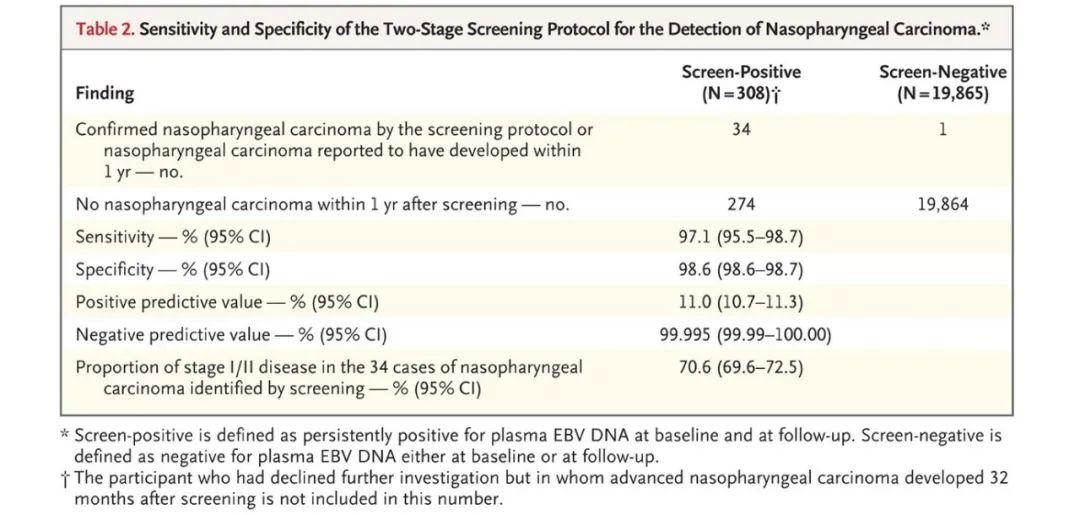
表1 基于cfEBV筛查鼻咽癌患者方法的特异性和敏感性
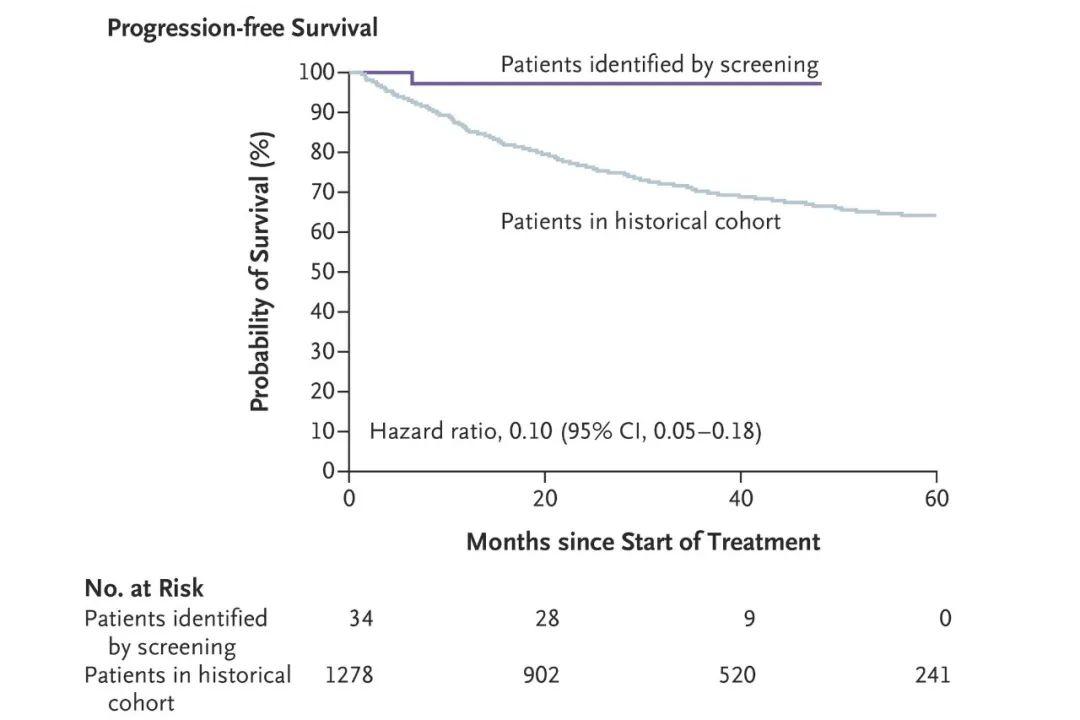
图2 该研究筛查出的34名鼻咽癌患者与2013年香港筛查人群中1278例患者的3年无进展生存率的比较。
小结:
液体活检已成为检测和监测早期和晚期癌症患者疾病状态的一种有前途的工具,现有数据也印证了液态活检生物标志物可用于头颈肿瘤的检测,以及在辅助治疗、手术治疗之后的局部复发、转移和预后评估。此外,cfDNA中与头颈肿瘤发病机制密切相关的生物标志物如HPV、EBV能够对特定的头颈肿瘤群体进行复发预后监测和早期筛查。而通过制定个体化的检测panel,在病程早期发现分子水平的复发,能够为预后评估和治疗提供指导,从而提高患者的生存质量。随着大规模的液态活检结合临床实践相关研究的开展以及头颈肿瘤完整的疾病信息图谱的构建,能够促使液态活检在HNSCC中的诊治有更广泛的应用,惠及更多的患者。
参考
- ^Schmidt, H., et al., The development of a liquid biopsy for head and neck cancers. Oral Oncol, 2016. 61: p. 8-11.
- ^孙岚, 液体活检在头颈部肿瘤诊治中的应用进展. 浙江医学, 2021. 43(06): p. 579-581+588.
- ^Economopoulou, P., et al., Liquid biopsy: An emerging prognostic and predictive tool in Head and Neck Squamous Cell Carcinoma (HNSCC). Focus on Circulating Tumor Cells (CTCs). Oral Oncol, 2017. 74: p. 83-89.
- ^Eder, T., et al., Interference of tumour mutational burden with outcome of patients with head and neck cancer treated with definitive chemoradiation: a multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group. Eur J Cancer, 2019. 116: p. 67-76.
- ^Plagnol, V., et al., Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS One, 2018. 13(3): p. e0193802.
- ^Tota, J.E., et al., Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J Clin Oncol, 2019. 37(18): p. 1538-1546.
- ^Chan, K.C., et al., Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res, 2003. 63(9): p. 2028-32.
原文地址:https://zhuanlan.zhihu.com/p/532699116 |
|
 /3
/3 