登陆有奖并可浏览互动!
您需要 登录 才可以下载或查看,没有账号?立即注册


×

诊断测试是许多领域的关键,这其中包括医疗保健和预防医学、食品安全和环境监测。因此,分析人员需要适合样品类型、分析物和测试环境的适当和准确的检测方法,并明确成本、时间和操作人员的限制。填补这一空白的测试之一是侧向层析测试(Lateral Flow Test, LFT)。在这篇文章中,我们将考虑什么是LFT,它们是如何工作的以及它们有哪些应用。
什么是侧向层析测试(LFT)?
侧向层析测试(LFT)是一种简单、快速和廉价的检测方法,可以检测液体样品中目标抗原或抗体的存在[1]。作为免疫测定的一种类型,检测依赖于抗体与其目标抗原的结合,结合检测反应,产生可读的、通常是可见的结果。
侧向层析测试的其他通用名称是什么?
LFTs有多个术语和缩写,其中一些是可以互换的,这取决于所指的是检测过程还是所用设备,以及检测的是抗原还是抗体,下表1总结了常见的术语。
表1 | 用于描述LFTs的名称和缩略语
| 术语 | 缩略语 | 备注 | | 侧向层析试验 | LFT | 可指测试过程或物理设备 | | 侧向层析测定 | LFA | 可指测试过程或物理设备 | | 侧向层析免疫测定 | LFIA | 可指测试过程或物理设备 | | 侧向层析试剂 | LFD | 指的是物理测试设备 | | 胶体金试纸 | - | 指的是物理测试设备 | | 抗原检测 | - | 可指任何测试抗原的试验,如酶联免疫吸附试验(ELISA),但包括LFTs | | 抗体检测 | - | 可指任何测试抗体的试验,如ELISA,但也包括LFTs。 | | 快速检测 | - | 虽然技术上不完全是LFT的术语,可以包括其他测试方式,但这个术语经常被认为是指LFT | | 快速抗原检测 | RAT或ART | 通常指测试目标抗原的LFT | | 快速抗体检测 | RAT或ART | 通常指的是测试目标抗体的LFT |
侧向层析免疫测定(LFIA)是如何工作的?
LFT通常被安置在一个塑料外壳内,有一个用于引入样品的孔和可以看到检测线和质控线的窗口。
进行直接LFT的过程如下(图1):
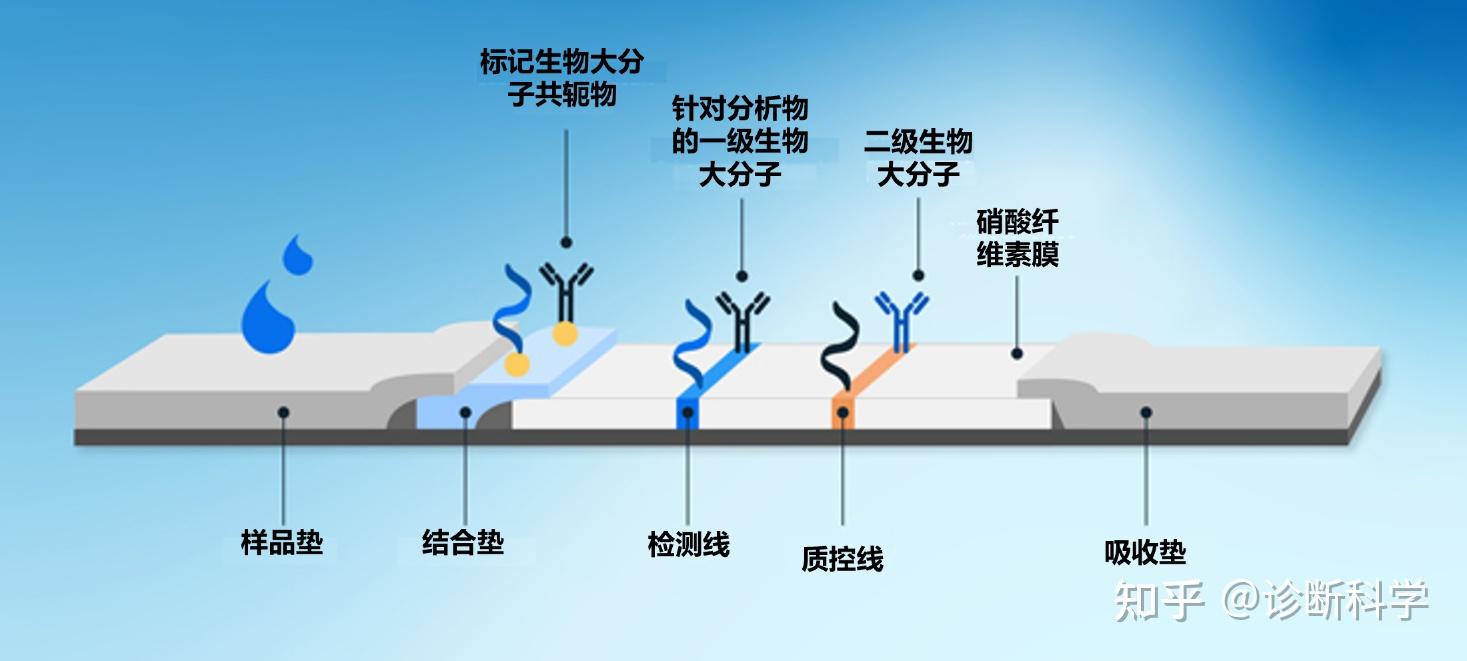
图1 | 侧向层析试剂(LFD)的示意图,显示了样品的主要组成部分和路径。
液体样品被引入位于样品井下方的样品垫并被吸收。样品量必须足以浸透样品垫,使样品沿着LFD膜渗入,但不能淹没装置,因此通常使用几滴样品。
通过毛细管作用,样品沿着设备被吸引到下一个垫子上,即所谓的结合垫。在这里,目标分析物(如果存在)和标记的免疫试剂之间发生结合。这通常是一种会与目标抗原结合的抗体或与某种形式的检测介质(如胶体金)结合的抗体。为了防止干扰检测线的结合,结合抗体被设计为与目标抗原和检测线抗体的不同区域结合。在检测抗体的地方,结合抗体通常与目标抗体的恒定区域而不是目标特异性可变区域结合。
样品继续沿着多孔膜(通常是硝化纤维素)流动。在这个过程中,结合的样品将通过膜上的检测线和质控线。在检测线上,待结合的目标分析物(如果存在的话)与膜上的免疫试剂发生结合。这个反应被设计成特异性的,因此只有在目标物存在的情况下才会发生结合。如果目标是一种抗原,检测线将包含固定的目标特异性抗体。如果目标是一种抗体,检测线将包含固定化的抗原,这些抗原是感兴趣的抗体的目标。
当样品到达质控线时,会发生结合,以表明结合物的释放和样品沿着膜的转移已经成功。这种结合反应通常是在固定化抗体和抗体结合物之间进行的,与样品分析物无关。因此,即使在没有目标分析物的情况下,也会出现一条质控线。
最后,剩余的样品流向LFD末端的吸收垫。
一旦使用了样品,就必须将测试放置一定的时间,以使结合和随后的检测反应发生。光学检测,特别是比色检测,是最经常使用的,因为结果可以用目测判读。然而,一些检测方法也被开发出来,比如使用荧光检测,但这需要专业的判读仪。生物化学检测反应,如基于辣根过氧化物酶的检测、表面增强拉曼光谱(SERS)、电化学和磁性[2]标签和检测方法也已被开发出来,但较少被使用[3]。使用的结合标签将决定所需的检测方式。
图2和图3分别显示了在直接LFTs中发生的检测抗原或抗体的结合反应的例子。
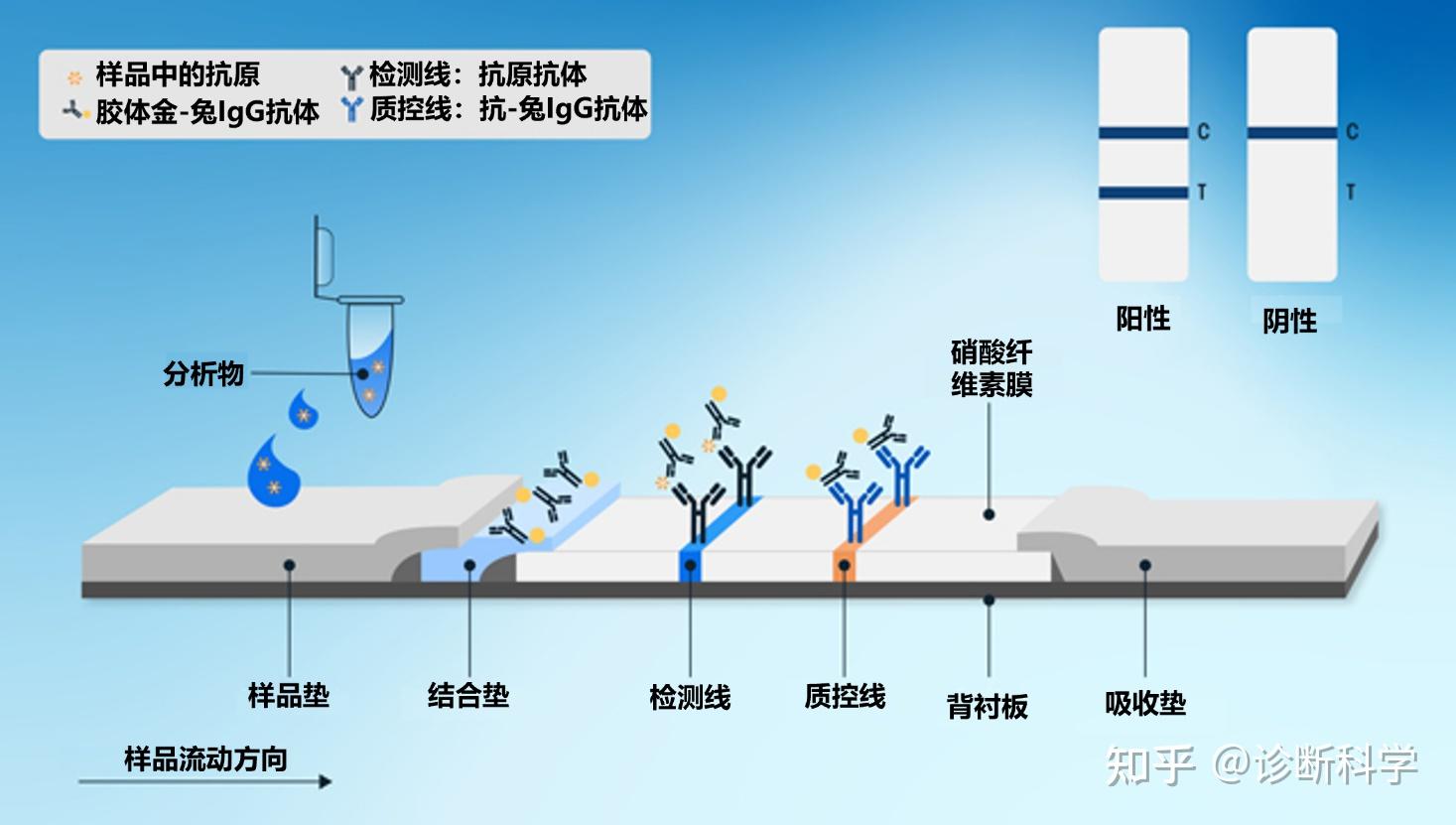
图2 | 检测样品中的抗原时,结合垫、检测线和质控线的结合反应。
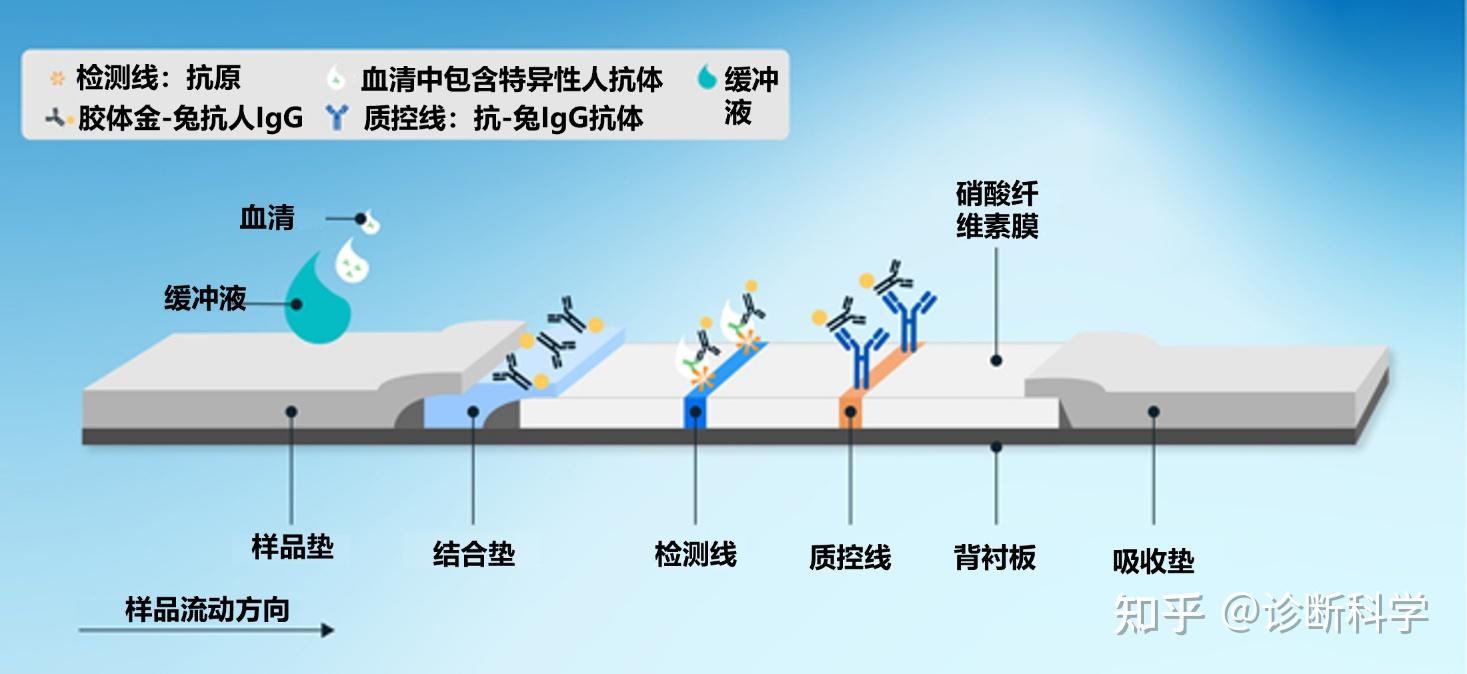
图3 | 检测样品中的抗体时,结合垫、检测线和质控线的结合反应。
如何判读侧向层析试剂(LFD)?
由于大多数检测都会产生可见的结果,在有或没有目标物的情况下,结合物会出现线条,因此许多LFT可以进行目测判读。直接检测成功的标志是质控线的存在,而阳性或阴性的结果则由检测线的存在或不存在来表示(图4)。另一种形式的LFT,即竞争性试验(我们将在下一节讨论)需要对结果进行不同的解释。尽管我们一般很少使用这类试剂,但确定我们正在评估哪种类型的测试是很重要的。
重要的是要注意,结果只应在为该特定测试规定的特定时间范围内进行解释。如果在引入样品后过早地读取,结果可能没有时间充分发展。如果在引入样品后放置过久,随着时间的推移,反应的恶化或过度发展,条带可能会发生改变。
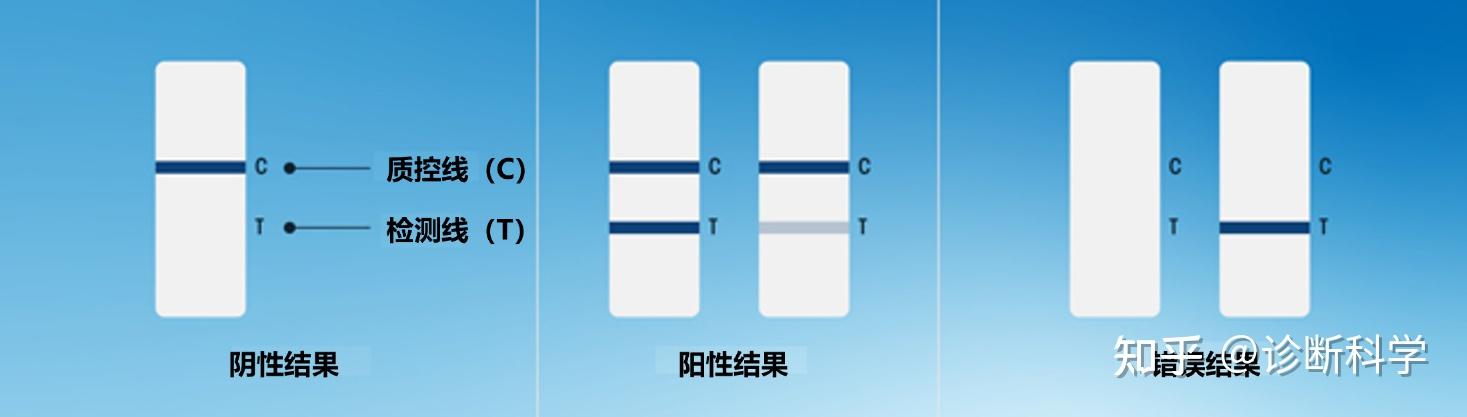
图4 | 直接解释LFT结果。图中显示
A)成功的测试提供了阴性结果;
B)成功的测试提供了强(左)弱(右)阳性结果;
C)无效测试的例子,其中质控线未能出现。
一些测试也可以使用手持式或台式扫描设备或智能手机以数字方式读取。第一批手持式判读仪在2000年开始投入商业使用[4]。对于产生荧光而非比色结果的测试,某种形式的判读仪是必要的。手持式设备和智能手机系统通常一次读取一个测试结果,而台式设备可以同时读取成批的LFD,但通常仅限于基于实验室的环境,与便携式设备不同。使用这些设备可以消除在读取、解释和(如适用)记录结果方面的人为错误的不确定性。一些判读仪还将测量检测线的强度,以提供一种定量的结果。当LFD上的线条很模糊时,数字测量可能特别有用,而且拥有测试结果的数字记录可以帮助解决数据完整性和可追溯性问题。它们还为医生收集病人监测数据提供了机会,可能会导致早期干预和更好的健康结果。
虽然台式和手持式判读仪通常对家庭使用不实用或负担不起,但智能手机判读仪填补了这个空白。早期的形式没有被广泛采用,因为需要额外的定制插件[5, 6, 7],以使手机能够捕获结果。然而,化验和手机技术的进步以及连接性的提高改善了这种情况。在新冠大流行期间,数字判读仪被批准使用,用户通过手机上的一个应用程序提交LFT的照片,人工智能被用来解释结果。创造者们希望该应用能更广泛地用于其他基于LFD的检验。随着对护理点和使用点定量检测的需求不断增加,传感器技术不断改进,尺寸和成本不断降低,我们似乎有可能继续看到这一领域的新技术发展[8]。
测试可以被设计为在一次运行中检测多个目标,即所谓的多重检测(在下一节中进一步讨论)[9]。在这种情况下,每个目标的结果都可以用一个共享的质控线和连续的检测线来表示,每个检测线都会显示样品对其相应的目标是阳性还是阴性(图5 A)。同样的原则也可用于创建一个阵列格式,即多个测试条,每个测试条针对一个目标并有自己的质控线,平行运行并从每个测试条上读取结果(图5 B)。

图5 | LFTs多重检测的物理安排。
A)目标依次出现在单个试纸上;
B)以阵列形式出现的单个试纸。
另外,也可以使用采用多个标签的系统,每个标签用于单个样品中的不同目标,这些标签可以相互区分,如具有不同发射特征的荧光标签(图6 A)。这种方法需要专业的判读仪。如果希望检测某一特定目标的整个类别,而不是区分特定目标(如某一病原体上的多种抗原或同一病原体的多种形式),可使用广义的选择性抗体,它将检测某一特定类别中一系列分子的存在,但作为单一的无差异结果报告(图6B)。

图6 | 多重LFT的免疫学探针选择,其中
A)在一次测试中使用多个探针,其信号可以被区分;
B)具有广泛选择性的抗体结合多个目标,并将其作为单一结果报告。
侧向层析测试的设计考虑
与大多数免疫测定一样,在开发LFT或将基于实验室的测定转移到LFD上时,有许多重要的考虑因素,仔细选择和优化所有的测定成分是很重要的。这些因素包括:
侧向层析测试的类型
我们将接触到的大多数LFT,实际上也是上面描述的类型,都是直接检测,然而,LFT可能是直接(夹心)或竞争(抑制)形式。直接检测通常用于检测具有多个抗原位点的较大的分析物,如人绒毛膜促性腺激素(hCG)的人类妊娠试验。在这里,结合物必须过量,以确保它和目标物一起,不会在检测线上全部被捕获,而被阻止到达质控线。
竞争形式通常用于测试具有单一抗原决定因素的小分子,这些小分子无法同时结合两种抗体。与直接检测相反,阳性结果是由没有检测线表示的,而在所有情况下仍应出现质控线。这是因为结合物已经与纯化的目标抗原结合,只有当样品中存在目标抗原时,结合物才会被竞争掉,并被阻止与检测线结合并产生结果(图7)。

图7 | 竞争性LFT中产生阳性或阴性结果的结合反应。
抗体
单克隆抗体结合其目标的单一表位,从而提供良好的特异性。使用多克隆抗体可以提高敏感性,因为它们将结合目标的多个表位,这与单克隆抗体不同,但有可能增加非特异性结合,降低特异性。无论使用单克隆抗体还是多克隆抗体,每种抗体都必须经过验证,并针对特定的检测方法进行优化,包括使用的数量,以确保准确和敏感的结果。
标签类型和相应的检测系统
光学读数在LFT中最常用,可细分为比色和荧光两组。相应的结合标签的选择将由检测的方式、地点和对象来指导。比色试验中最常使用的标签包括胶体金、纳米粒子、纤维素和聚合物珠,而荧光标签包括荧光珠[10]和量子点[11]。
样品垫
样品垫[1]必须能够吸收样品并以与LFD兼容的形式释放。因此,它必须有足够的吸收能力,但同时又有适合特定样品类型的特性。这可能意味着需要过滤掉红细胞或微粒,改变pH值或破坏基质成分,这可以通过对垫子的适当预处理来实现。
结合垫
结合垫[1]必须能够保持结合物,并在LFD的使用寿命内保持稳定,在使用LFD时有效而可靠地释放。不良或不均匀的干燥和释放可能是检测性能变化的重要来源。因此,在装载结合垫时,优化材料、涂层和干燥过程是很重要的。同样重要的是,选择的材料不能阻碍样品的流动。
膜
膜[1]必须能够让样品/缓冲液和结合物稳定地流过,同时在LFD的生命周期内将免疫试剂稳定地保持在检测线和质控线上。它还必须不干扰结合反应或选择的检测方法。硝化纤维素是一种流行的材料选择;其他材料也被尝试过,但一般都不太成功。
吸收垫
膜所能容纳的体积是有限的,因此吸收垫[1]的作用是增加通过膜的样品量,从而增加目标物(如果存在的话)通过检测线的量,提高检测的敏感性和特异性。它通过吸收到达它的样品(和缓冲液,如果使用的话)来实现这一目的,使更多的样品通过膜被吸收。吸收垫的床层体积必须超过要运行的样品/缓冲液,以便能够保持多余的体积,直到可以读取结果,而不允许有潜在的干扰性回流。因此,选择正确的厚度、密度、强度和材料很重要。由于纤维素的吸收能力,它是一个受欢迎的选择。
样品
样品本身是成功的LFT的一个重要考虑因素。非常粘稠的样品可能无法通过膜或导致堵塞,从而导致测试失败,而不均匀的样品可能产生不可靠的结果。样品浓度也是一个重要的考虑因素。太稀了,可能会错过阳性结果,太浓了,可能会干扰测试性能。因此,一些样品类型可能需要预处理(如粘液溶解剂)、分离(如从全血中提取血清)或稀释,使其适合基于LFD的测试,并产生在测试动态范围内的结果。
多重检测
虽然检测一个目标就足够了,但一些应用从多重检测中获益,以便在一次检测中检测多个目标或同一总体目标的多个部分。这就避免了对同一样品的重复检测,从而减少了时间和成本。然而,在一个设备上加入多个检测反应会增加检测优化的复杂性,避免不同目标之间的交叉反应或干扰很重要。多条检测线的空间分离是一种流行的多重检测方式,尽管与单一目标检测相比,它可能使结果的解释复杂化,需要更多的材料和样品,并增加结果的时间。阵列形式克服了组合优化、运行时间和读取紧密间隔的结果的问题,但也需要明显更多的材料和样品。采用可区分标签的多重检测策略通常也需要专门的读取者来解释结果。
侧向层析测试的优势和劣势
LFTs有很多优点,导致其被广泛使用,特别是在怀孕测试和COVID-19大流行病中的应用。然而,与大多数检测方法一样,它们的使用也有相关的限制[12],总结如下。
优势:
- 便宜;
- 快速得到结果(通常是几分钟);
- 携带方便,尤其是那些可以用目视判读的检测方法;
- 易于使用;
- 通常可以在环境温度下保存,对于护理点和家庭测试或在偏远地区的测试非常有用,因为那里的制冷可能很困难或不稳定;
- 通常有很长的保质期;
- 大多数不需要电源;
- 一系列不同的数字判读仪提供了适合不同环境的选择;
- 所需样本量小;
- 判读仪可提高结果解释/记录的准确性;
- 可多重检测。
劣势:
- 含有微粒/粘性的样品可能导致设备堵塞或运行不稳定;
- 可能需要对某些类型的样品进行预处理;
- 准确度可能低于基于实验室的检测(灵敏度/特异性较低);
- 一旦测试完成,必须对可能含有传染性物质的样品进行适当的处理,这在护理环境之外可能更具挑战性,且执行力较差;
- 尽管一些台式判读仪可以接受多个设备,但LFT通常不太适合高通量分析;
- 通常需要在有限的时间内读取,因为如果放置时间过长,结果会褪色或过度发展;
- 给出一个“是”或“否”的答案,通常不是定量的(数字读取设备除外),所以会妨碍对感染阶段/对他人构成的风险等的解释;
- 如果使用台式或手持设备而不是智能手机,判读仪会增加检测开发的复杂性和用户的成本;
- 再现性可能因批次不同而不同;
- 交叉反应可能是一个挑战,特别是对于多重检测试剂而言
侧向层析测定(LFA)的常见应用及其目标
LFT的应用是如此之多和多样化,以至于不可能在这里全部涵盖,但下面是最受欢迎的使用领域的一个选择。
妊娠试验目标
LFT最著名的例子之一可能是家庭妊娠试验。它从20世纪80年代开始出现[13],检测尿液中由怀孕期间胎盘产生的hCG。多年来,灵敏度有所提高,现在的测试通常会在受孕后10天或11天提供一个阳性结果。
家庭新冠测试
大多数人可能已经亲身经历过一次或多次的家庭新冠抗原测试[14]。人们在家里使用的大多数设备都是为了检测病毒本身的存在(即,他们可能会被感染吗?),而不是检测表明暴露或接种的抗体。因此,它们的方向是与病毒的暴露和可接触的抗原部分结合,如尖峰、包膜、膜或核壳蛋白[15]。
人类病原体
虽然SARS-CoV-2可能是我们想到的第一个人类病原体,但LFTs在检测一系列目标方面也有用处,包括导致疟疾的疟原虫、结核分枝杆菌(结核病的致病因子)[16]、乙肝病毒[17]和人类免疫缺陷病毒(HIV)[18]。
动物病原体
受益于LFTs的也不仅仅是人类医学。除了在动物中进行妊娠检测外,还可以用这种形式检测一些兽医病原体。它对在诊所以外工作的从业人员或农民、饲养员或业主的测试特别有用。这包括非洲猪瘟[19]和牛肠道病原体[20]。LFT检测在根除牛瘟的过程中起到了关键作用[21]。农场检测也有助于防止食品生产动物中的病原体到达消费者手中。但是,对一些疾病(如狂犬病)的检测质量提出了批评[22]。
食品污染物
食品污染物有多种形式,从病原体和杀虫剂到重金属和毒素。黄曲霉毒素是由黄曲霉和寄生曲霉产生的次级代谢物,存在于谷物类食品、坚果和香料中,就是这样一个例子[23]。它们涵盖了一组化学上相关的化合物,针对这些毒素,已经开发了许多LFTs。由于其体积小,黄曲霉毒素的LFTs通常是间接形式。还开发了检查黄曲霉毒素和其他霉菌毒素的多重测试[24]。沙门氏菌是常见的食品病原体,对其进行快速诊断有助于加快治疗,确定污染源并有助于防止进一步传播[25]。LFT技术提供了一种手段,利用噬菌体技术来确定测试条的有效粘合剂[26],甚至可以区分活的和死的肠炎沙门氏菌。
环境污染物
检测环境污染物,包括杀虫剂、双酚A(BPA)和重金属,对于保护人类、动物和环境非常重要。便携式检测解决方案使分析人员能够在现场发现问题并迅速采取行动。已经开发了能够检测汞[27]、铬[28]和镉[29]离子的LFTs。BPS,一种令人担忧的干扰内分泌的化合物,也可以用这种技术来检测,并且与基于实验室的测试相比,显示出良好的结果[30]。有机磷农药可以用间接LFTs检测[31],同时还开发了一种同时检测水中克百威和三唑磷的试验[32]。
过敏原
患有过敏症的人必须小心翼翼地确保避开他们的诱发因素。同样,食品生产商也必须确保宣布为“无”的产品是真正的。LFTs提供了一种快速而简单的方法来保证人们的安全,并且已经针对一系列常见的过敏原进行了开发,包括麸质[33]、酪蛋白[34]、大豆[35]、芥末和各种坚果[36]。
需要密切监测的治疗性药物
对于一些治疗性药物,在无效剂量、最佳剂量和超过安全水平(可能出现严重的不良反应)之间存在着细微的差距。为了确保病人保持在最佳剂量范围内,需要进行密切监测,辅助诊断,如LFTs可以提供这种监测。地高辛是一种用于治疗心动过速的强心剂,就是这样一种药物,科学家们开发了一种可以用智能手机应用程序读取的LFT,以帮助病人进行自我监测和用药[37]。
滥用药物
尿液分析(LFA非常适合)仍然是英国最常见的非法药物测试方法之一,为工作场所测试或执法等情况提供快速结果。还开发了一种可卡因的检测方法,与许多滥用药物的检测方法不同,由于可卡因的分子量小,所以采用了竞争性的形式,通过采用仿生材料,采用非竞争性的形式[38]。甚至已经开发出可以在指纹的汗液中检测出Δ9-四氢大麻酚、可卡因、阿片剂和苯丙胺的LFAs[39]。
诊断科学编辑团队收集、整理和编撰,如需更多资讯,请关注公众号诊断科学(DiagnosticsScience)。
参考文献
- O’Farrell B. Evolution in lateral flow–based immunoassay systems. Lat Flow Immuno. Published online October 31, 2008:1-33. doi:10.1007/978-1-59745-240-3_1
- Moyano A, Serrano-Pertierra E, Salvador M, Martínez-García JC, Rivas M, Blanco-López MC. Magnetic lateral flow immunoassays. Diagnostics (Basel). 2020;10(5):288. doi:10.3390/diagnostics10050288
- Bahadır EB, Sezgintürk MK. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016;82:286-306. doi:10.1016/j.trac.2016.06.006
- Faulstich K, Gruler R, Eberhard M, Lentzsch D, Haberstroh K. Handheld and portable reader devices for lateral flow immunoassays. In: Wong R, Tse H, eds. Lateral Flow Immunoassay. Humana Press; 2009:1-27. doi:10.1007/978-1-59745-240-3_9
- Zangheri M, Cevenini L, Anfossi L, et al. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015;64:63-68. doi:10.1016/j.bios.2014.08.048
- Mudanyali O, Dimitrov S, Sikora U, Padmanabhan S, Navruz I, Ozcan A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip. 2012;12(15):2678-2686. doi:10.1039/C2LC40235A
- You DJ, Park TS, Yoon JY. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosens Bioelectron. 2013;40(1):180-185. doi:10.1016/j.bios.2012.07.014
- Park J. Lateral flow immunoassay reader technologies for quantitative point-of-care testing. Sensors. 2022;22(19):7398. doi:10.3390/s22197398
- Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C. Multiplex lateral flow immunoassay: An overview of strategies towards high-throughput point-of-need testing. Biosensors (Basel). 2018;9(1):2. doi:10.3390/bios9010002
- He F, Lv X, Li X, Yao M, Li K, Deng Y. Fluorescent microspheres lateral flow assay integrated with smartphone-based reader for multiple microRNAs detection. Microchem. J. 2022;179:107551. doi:10.1016/j.microc.2022.107551
- Bock S, Kim HM, Kim J, et al. Lateral flow immunoassay with quantum-dot-embedded silica nanoparticles for prostate-specific antigen detection. Nanomaterials (Basel). 2021;12(1):33. doi:10.3390/nano12010033
- Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569-582. doi:10.1007/s00216-008-2287-2
- Leuvering JHW, Goverde BC, Thal PJHM, Schuurs AHWM. A homogeneous sol particle immunoassay for human chorionic gonadotrophin using monoclonal antibodies. J Immunol. Methods. 1983;60(1):9-23. doi:10.1016/0022-1759(83)90330-7
- Peto T, Affron D, Afrough B, et al. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. eClinicalMedicine. 2021;36. doi:10.1016/j.eclinm.2021.100924
- Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.587269
- Ariffin N, Yusof NA, Abdullah J, et al. Lateral flow immunoassay for naked eye detection of Mycobacterium tuberculosis. J. Sens. 2020;2020:e1365983. doi:10.1155/2020/1365983
- Song LW, Wang YB, Fang LL, et al. Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Anal Chem. 2015;87(10):5173-5180. doi:10.1021/ac504832c
- Turbé V, Herbst C, Mngomezulu T, et al. Deep learning of HIV field-based rapid tests. Nat Med. 2021;27(7):1165-1170. doi:10.1038/s41591-021-01384-9
- Onyilagha C, Nguyen K, Luka PD, et al. Evaluation of a lateral flow assay for rapid detection of African swine fever virus in multiple sample types. Pathogens. 2022;11(2):138. doi:10.3390/pathogens11020138
- Cho YI, Sun D, Cooper V, Dewell G, Schwartz K, Yoon KJ. Evaluation of a commercial rapid test kit for detecting bovine enteric pathogens in feces. J VET Diagn Invest. 2012;24(3):559-562. doi:10.1177/1040638712440997
- Brüning A, Bellamy K, Talbot D, Anderson J. A rapid chromatographic strip test for the pen-side diagnosis of rinderpest virus. J Virol Methods. 1999;81(1):143-154. doi:10.1016/S0166-0934(99)00068-3
- Klein A, Fahrion A, Finke S, et al. Further evidence of inadequate quality in lateral flow devices commercially offered for the diagnosis of rabies. Trop Med Infect Dis. 2020;5(1):13. doi:10.3390/tropicalmed5010013
- Anfossi L, Baggiani C, Giovannoli C, et al. Lateral flow immunoassays for aflatoxins B and G and for aflatoxin M1.In: Razzaghi-Abyaneh M ed. Aflatoxins. IntechOpen; 2013. doi:10.5772/51777
- Yu S, He L, Yu F, et al. A lateral flow assay for simultaneous detection of deoxynivalenol, fumonisin B1 and aflatoxin B1. Toxicon. 2018;156:23-27. doi:10.1016/j.toxicon.2018.10.305
- Çam D. Lateral flow assay for Salmonella detection and potential reagents. In: Ranjbar M, Nojomi M, Mascellino MT. New Insight into Brucella Infection and Foodborne Diseases. IntechOpen; 2019. doi:10.5772/intechopen.88827
- Charlermroj R, Makornwattana M, Phuengwas S, Karoonuthaisiri N. A rapid colorimetric lateral flow test strip for detection of live Salmonella Enteritidis using whole phage as a specific binder. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.1008817
- He Y, Zhang X, Zeng K, et al. Visual detection of Hg2+ in aqueous solution using gold nanoparticles and thymine-rich hairpin DNA probes. Biosens. Bioelectron. 2011;26(11):4464-4470. doi:10.1016/j.bios.2011.05.003
- Liu X, Xiang JJ, Tang Y, et al. Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Anal. Chim. Acta. 2012;745:99-105. doi:10.1016/j.aca.2012.06.029
- López Marzo AM, Pons J, Blake DA, Merkoçi A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens. Bioelectron. 2013;47:190-198. doi:10.1016/j.bios.2013.02.031
- Mei Z, Qu W, Deng Y, et al. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples. Biosens. Bioelectron. 2013;49:457-461. doi:10.1016/j.bios.2013.06.006
- Du D, Wang J, Wang L, Lu D, Lin Y. Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: Biomarker of exposure to organophosphorus agents. Anal Chem. 2012;84(3):1380-1385. doi:10.1021/ac202391w
- Guo YR, Liu SY, Gui WJ, Zhu GN. Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 2009;389(1):32-39. doi:10.1016/j.ab.2009.03.020
- Hnasko RM, Jackson ES, Lin AV, Haff RP, McGarvey JA. A rapid and sensitive lateral flow immunoassay (LFIA) for the detection of gluten in foods. Food Chem. 2021;355:129514. doi:10.1016/j.foodchem.2021.129514
- Galan-Malo P, Pellicer S, Pérez MD, Sánchez L, Razquin P, Mata L. Development of a novel duplex lateral flow test for simultaneous detection of casein and β-lactoglobulin in food. Food Chem. 2019;293:41-48. doi:10.1016/j.foodchem.2019.04.039
- Gautam PB, Sharma R, Lata K, Rajput YS, Mann B. Construction of a lateral flow strip for detection of soymilk in milk. J Food Sci Technol. 2017;54(13):4213-4219. doi:10.1007/s13197-017-2890-3
- Le QN, Vance A, Bakir N, et al. Validation of the Reveal® 3-D for peanut lateral flow test: AOAC performance tested method SM 111901. J AOAC Int. 2020;103(4):1112-1118. doi:10.1093/jaoacint/qsz041
- Ruppert C, Phogat N, Laufer S, Kohl M, Deigner HP. A smartphone readout system for gold nanoparticle-based lateral flow assays: application to monitoring of digoxigenin. Microchim Acta. 2019;186(2):119. doi:10.1007/s00604-018-3195-6
- Guler E, Yilmaz Sengel T, Gumus ZP, et al. Mobile phone sensing of cocaine in a lateral flow assay combined with a biomimetic material. Anal Chem. 2017;89(18):9629-9632. doi:10.1021/acs.analchem.7b03017
- Hudson M, Stuchinskaya T, Ramma S, et al. Drug screening using the sweat of a fingerprint: lateral flow detection of Δ9-tetrahydrocannabinol, cocaine, opiates and amphetamine. J. Anal. Toxicol. 2019;43(2):88-95. doi:10.1093/jat/bky068
***
原文地址:https://zhuanlan.zhihu.com/p/618479771 |
 /3
/3 