登陆有奖并可浏览互动!
您需要 登录 才可以下载或查看,没有账号?立即注册


×
何为微流控?
微流控是一种精确控制和操控微尺度流体的技术,尤其特指亚微米结构的技术。微,意味着具备以下特性:
l 微小的容量(纳升,皮升级别)
l 微小的体积
l 低能量的消耗
l 装置本身专用体积小
微流控在20世纪80年代兴起,并在DNA芯片,芯片实验室,微进样技术,微热力学技术得到了发展。
微流控研究的空间特征尺度范围在1微米至1毫米。
微流控应用范围
微流体结构包括微气体系统,即用于处理片外流体(液体泵,气体阀等)的微系统,以及用于片上处理纳升(nl)和皮升(pl)体积的微流体结构。迄今为止,最成功的的微流体的商业应用是喷墨打印头。此外,微流体制造的进步允许以低成本塑料生产设备,并且可以自动验证部件质量。
微流体技术的进步正在革新分子生物学方法进行酶分析(如葡萄糖和乳酸分析),DNA分析(如聚合酶链式反应和高通量测序)和蛋白质组学。微流体生物芯片的基本思想是将检测操作,以及样品预处理和样品制备在一个芯片上进行整合。
生物芯片的新兴应用领域是临床病理学,特别是疾病的即时现场诊断。此外,能够对生化毒素和其他危险病原体的空气/水样进行连续采样和实时测试的基于微流体的设备可以作为一个永远在线的“生物烟雾报警器”进行预警。
微流控技术已经为生物学家创造了强大的工具来控制整个的细胞环境,从而导致新的问题和新的发现。
该技术在微生物学方面的多种优势如下所示:
l 一般单细胞研究包括生长
l 细胞衰老:诸如“母机”之类的微流体装置允许跟踪数千个单个细胞数代,知道它们死亡
l 微环境控制:从机械环境到化学环境
l 通过在当个设备中引入多个化学输入来确定精确的时空浓度梯度
微流控的几个应用范例
- 基于微流控等温扩增技术的甲型H1N1流感病毒快速检测
目的:以甲型H1N1流感病毒检测为例,建立一种简便、快速的流感病毒检测方法,为呼吸道传染病疫情防控提供技术
方法:整合环介导等温扩增技术(LAMP)和微流控芯片技术,建立微流控实时荧光逆转录环介导等温扩增(RT-LAMP)快速检测犯法,针对甲型H1N1流感病毒的M基因序列中8个不同区段设计LAMP引物,根据扩增效果筛选出1套(6条)引物,并进行临床样本检测验证。
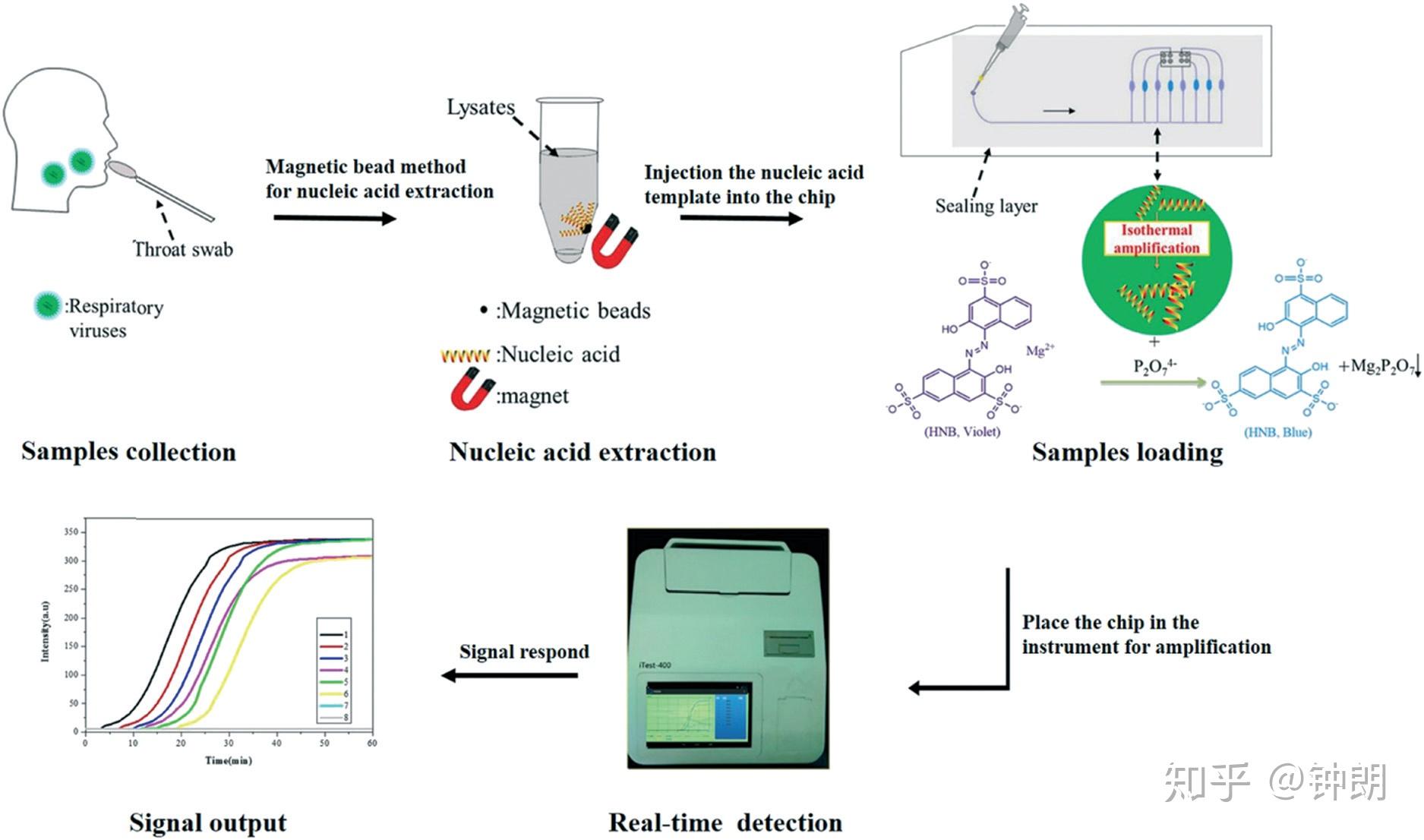
Schematic illustration of the strategy used to detect multiple respiratory viruses with a LAMP-integrated microfluidic chip system formultiplexed respiratory virus assays (LMCS-MRVAs).
平台设计:微流控芯片的上层为芯片密封层盖,避免产生液体蒸发形成气溶胶污染,下层为由聚碳酸酯(PC)和聚乙烯(PE)材料制作的十通道反应芯片。采用光纤传感器根据吸光原理建立了微流控RT-LAMP芯片。将光纤发射探头和接收头对插于LAMP扩增池中,柱面镜非球面镜组成的激光光源模块,经过扩增池后由物镜和滤光片组成光源接收模块接受,通过微型传感器后,将扩增池中的光信号转变为数字信号。
The measurement system that we developed was hand-held, with a size of 41 × 31× 12 cm and a weight of 2.7 kg. The schematic drawing of the measurement system is shown in Fig. S1.‡ The instrument was mainly composed of the heating module, the detection module, the control and processing display module and the outer cover. The heating module consists of a heating film and a heating machine. The detection module is composed of a high-definition camera, a light source and a fixed structure. The control and processing display module is composed of a circuit board, an ARM board and a screen. The instrument used a double-row array of white LEDs as the light source and the detector was a high-definition color camera. During the LAMP, the RGB color values in each reaction microchamber were obtained by an image recognition algorithm, and the blue component was taken as the real-time curve value to draw the real-time LAMP curve. The time required for the real-time LAMP signal to exceed the threshold signal was used for quantitation, similar to the threshold cycle number (Ct) in real-time PCR. Based on the experimental results, the negative signal intensity was less than 5, so the positive signal intensity was set to 15, i.e., three times the signal-to-noise ratio. To define the start time of amplification, we drew a straight line parallel to the abscissa with a signal value of 15 on the ordinate, and added a perpendicular line from the abscissa with the point intersecting the amplification curve. The intersection with the abscissa represented the amplification start time. With this detection system, the eight-channel microfluidic chip was disposable, and the realtime detection instrument can be reused.
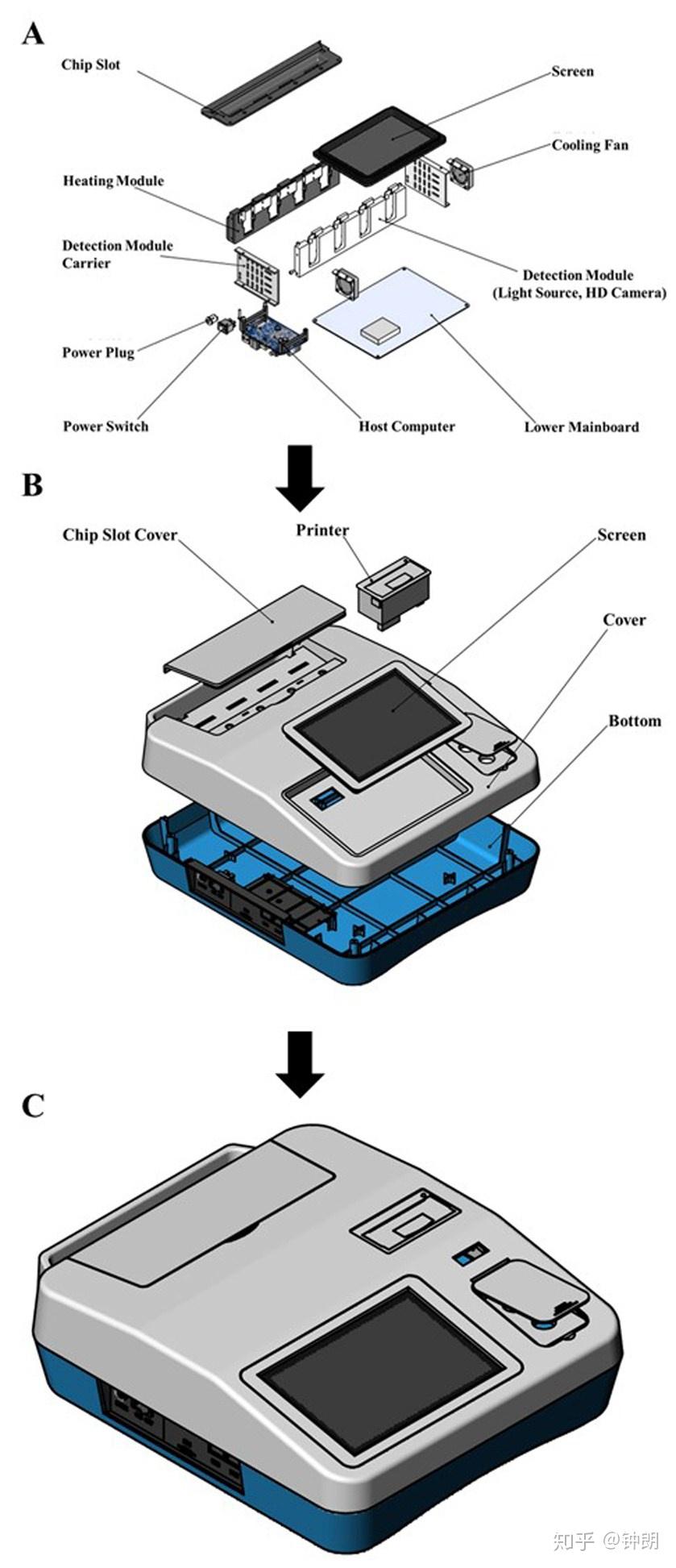
Fig. S1. The schematic drawing of the measurement instrument, A represent internal structure ofthe instrument, B represent external structure of the instrument, and C represent the detectioninstrument.
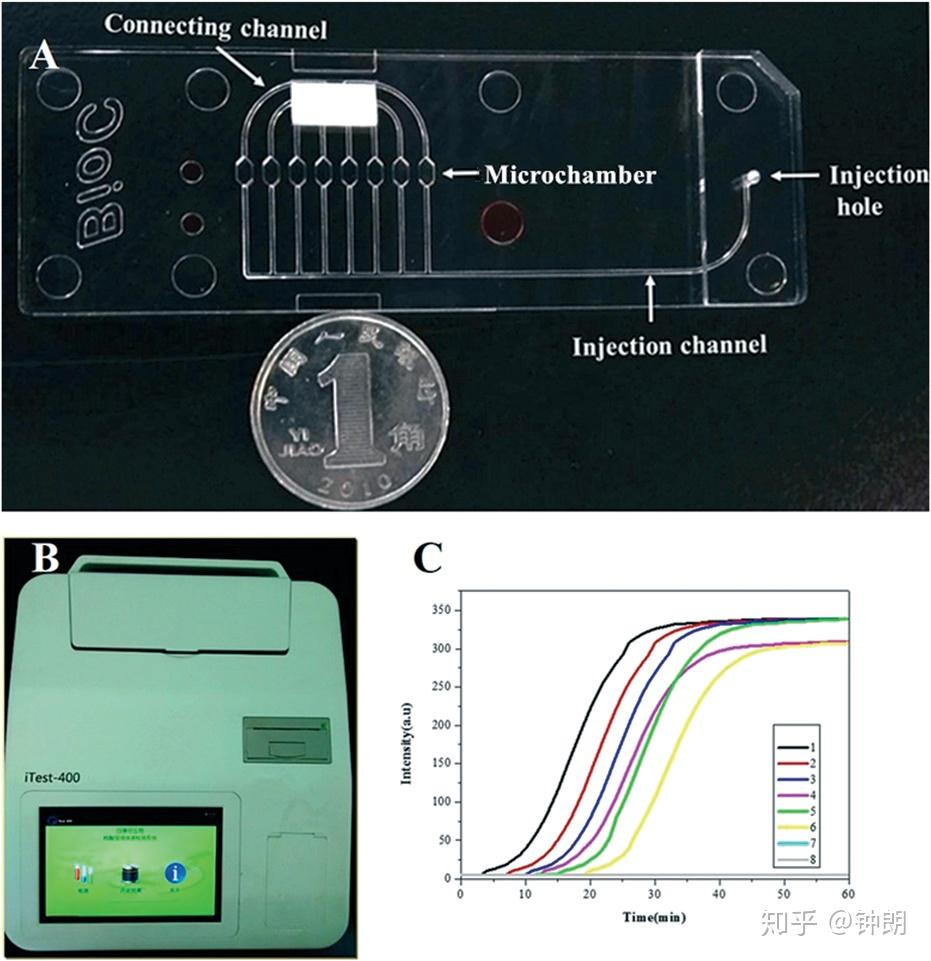
The developed microfluidic chip, real-time detection instrument,and representative real-time amplification curves. (A) Photographof a microfluidic chip. (B) Real-time detection instrument. (C)Real-time amplification curves.
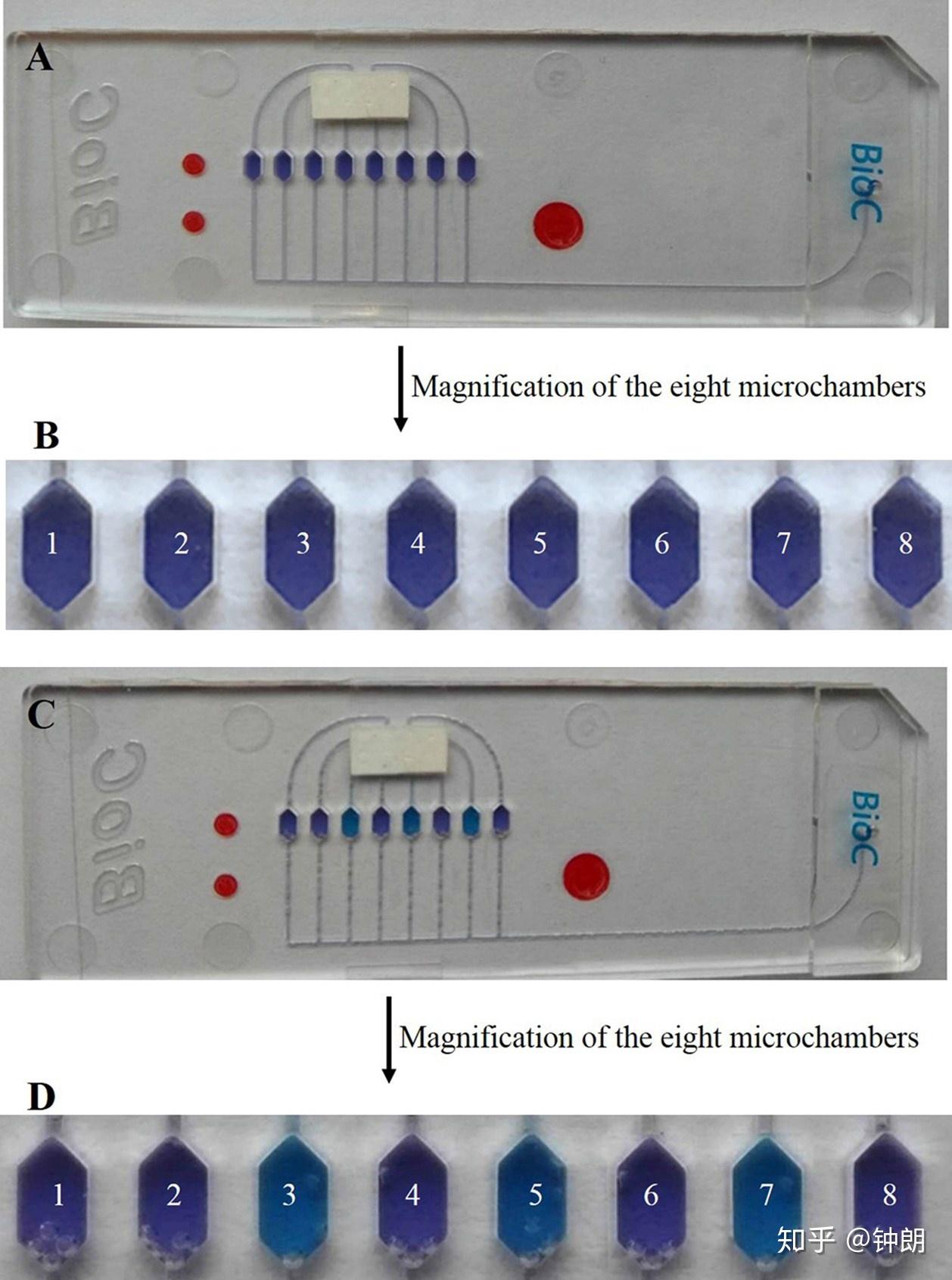
Fig. S2. Colorimetric change of microfluidic chip after reaction. (A) The whole microfluidic chipbefore the reaction. (B) Magnification of the eight microchambers before the reaction. (C) The whole microfluidic chip after the reaction. (D) Magnification of eight microchambers aft
芯片设计:The chip was produced using an internal injection-molding method using polycarbonate. The microfluidic chip contained eight independent reaction microchambers (2 × 1 × 1 mm), with a corresponding reaction volume of approximately 2 μl. The microchip microchannels had a width of about 500 μm and a depth of 200 μm, and flowed through the injection hole, microchambers, and connecting channel. The microfluidic chip was sealed with a polymer film with a thickness of 200 μm, which could be appropriately pressurized to ensure that the chip was sealed with no bubbles. After the nucleic acids were extracted from the samples, the nucleic acids were mixed with reaction solution, and the mixture was added to the microchambers of the microfluidic chip using a micropipette. Each solution was then diverted into the eight microchambers along the different channels and sealed with a film to ensure that the LAMP was carried out within a sealed chip. As LAMP proceeded, the color of the reaction system gradually changed from violet to sky blue, and the detection instrument developed in this study drew a real-time amplification curve based on the colorimetric change.
芯片操作流程:LAMP-integrated microfluidic chip system. All the solutions were prepared using ultrapure DNase/RNase-free distilled water. With each respiratory virus, the LAMP primers were used at a concentration of 1.6 μM each for FIP and BIP, 0.2 μM each for F3 and B3, and 0.8 μM each for LF and LB. Random primers were used as a negative control. Each corresponding LAMP primer mixture and negative control were embedded in the eight different microchambers of the microfluidic chip in advance (1: H1N1 primers, 2: H3N2 primers, 3: H5N1 primers, 4: H7N9 primers, 5: FluB primers, 6: HAdV primers, 7: HAdV primers, and 8: negative control) and then dried before sealing. Seventy microliters of the whole reaction system contained 5 μl of the nucleic acid sample to be tested extracted with the homemade magnetic beads. The reaction mixture also included 7.5 μl ThermoPol buffer, 4.5 μl MgSO4 (120 mM), 10.5 μl dNTPs (10 mM), 3 μl AMV reverse transcriptase (1000 units), 4 μl betaine (250 mM), 1 μl HNB (20 mM), 3 μl Bst 2.0 WarmStart® DNA polymerase (8000 units), and 31.5 μl DNase/RNase-free distilled water. The reaction system was mixed and injected into the injection hole of the chip to the separate microchambers through the eight microchannels. Then, the injection port and channels were resealed. Finally, the chips were inserted into the detection instrument. The reaction temperature was preset to 65 °C for 60 min for isothermal amplification and 80 °C for 10 min to terminate the reaction. HNB served as a metal ion indicator in the system. Initially, Mg2+ binding to HNB caused the color of the reaction system to be violet. As the reaction proceeded, Mg2+ reacted with precipitated pyrophosphate ions to form the magnesium pyrophosphate precipitate, such that HNB lost the bound Mg2+, making the system color become sky blue (Fig. S2‡). In the absence of LAMP, the system maintained a violet color. In order to reduce the influence of bubbles on the LAMP, we treated the reagents with an ultrasonic procedure before amplification, and compared every microchamber with the negative control microchamber to give the amplification curve. Moreover, we used the developed LMCS-MRVA system to detect those six kinds of respiratory virus, and used the gel electrophoresis assay to compare the detection results (Fig. S3‡).
结果:所建立的检测方法可实现扩增结果的荧光实时判读,能在恒定温度(65℃)30min内完成扩增反应,检测限可达10 copies/微升,仅对甲型H1N1流感病毒产生扩增曲线,对甲型H3、H5、H7、H9及乙型流感病毒均无扩增;对70份临床样本的检测结果与荧光定量PCR方法一致,特异性和灵敏度均为100%;该方法具有体系封闭、污染小、试剂用量小的优势。
原文地址:https://zhuanlan.zhihu.com/p/59379404 |
 /3
/3 